Balancing Drug Innovation and Marketing Competition
KEY TAKEAWAYS
- Patents and other protections for pharmaceutical innovation give inventors the incentive to develop new drugs, improving the health of American patients.
- Patents last for 20 years, beginning at the date the manufacturer files the application. Drug makers sometimes use tactics to delay less expensive alternatives from entering the market.
- The HELP and Judiciary committees have advanced bills to address anti-competitive behavior and encourage generic drug competition.
The federal government protects the rights of prescription drug inventors through various means, including patents. The goal is to give manufacturers incentives to create innovative products that benefit people. These protections must be balanced against another public good: the lower prices that come with competition from other companies selling a generic form of the original branded drug. In June, the Senate Judiciary Committee and the Health, Education, Labor and Pensions Committee both advanced bills intended to address anti-competitive behavior, to increase the number of generic drugs, and to lower drug costs.
FDA Review of Generic Drug Applications
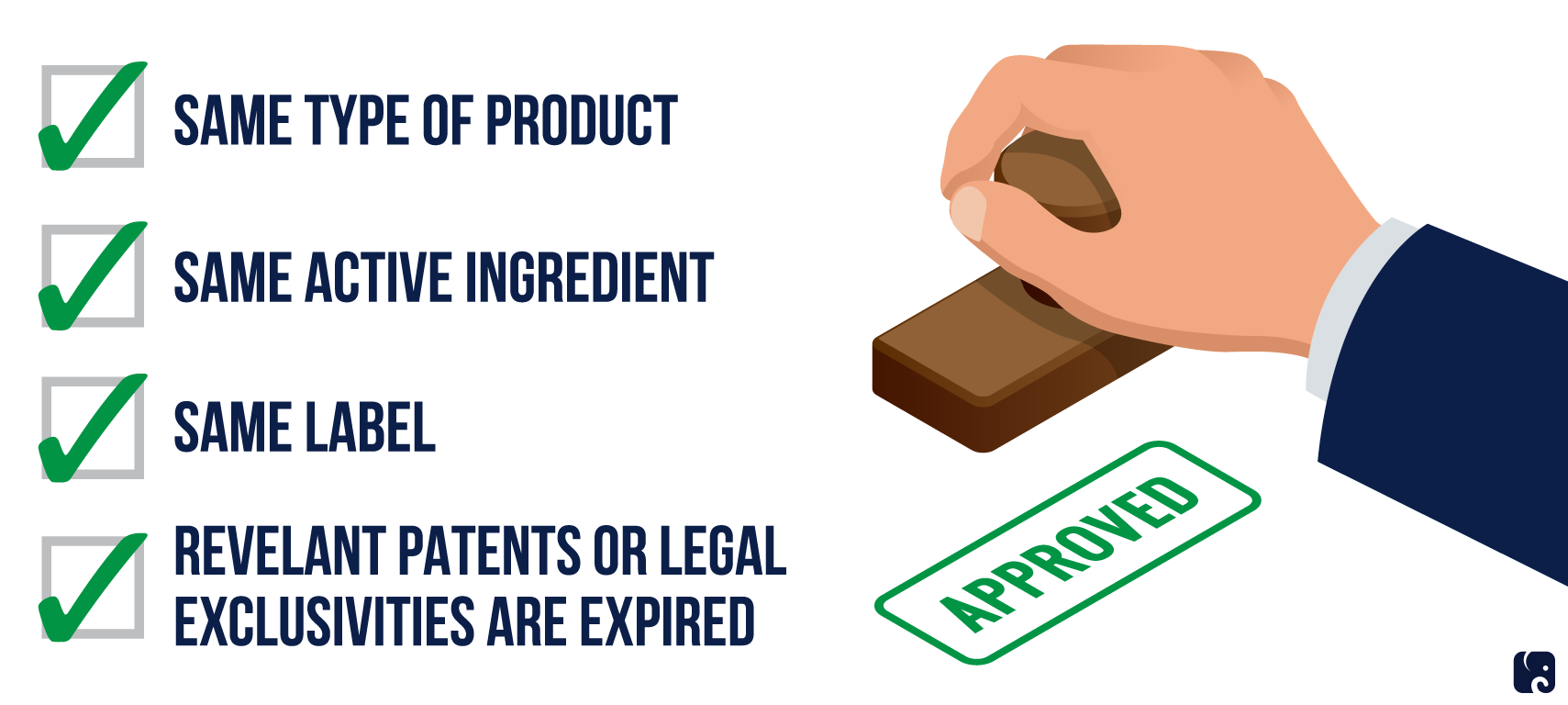
protections for new drugs
New pharmaceuticals can be incredibly expensive to develop and require many years of research and testing. Few ideas make it through the entire process and reach pharmacy shelves. The U.S. Patent and Trademark Office awards patents to branded products that are proven to be “novel,” “nonobvious,” and useful to the public. Patent terms are set by statute and currently last for 20 years, beginning at the date of patent application. The formulation patent is usually filed very early in the drug development process. The patent length can be extended under certain circumstances.
Patents provide the owner with the right to keep others from making the same drug formulation, using a specific drug delivery device, or manufacturing process for a specific period of time. As a result, the patent holder can maintain higher product prices and bring in significantly higher revenues than otherwise would have been possible if there was competition.
In addition to patents, the U.S. Food and Drug Administration can protect new pharmaceuticals by giving manufacturers “market exclusivity” upon approval. The drug’s creator gets the sole right to sell a drug – typically lasting three to seven years, depending on the type of drug – before other companies can offer a generic version at lower cost.
speeding up the approval of generic drugs
In 1984, Congress passed the Drug Price Competition and Patent Term Restoration Act, known as the Hatch-Waxman Act, to increase generic drug approval and competition. Prior to this law, a manufacturer that wanted to sell a generic version of a drug had to go through the same lengthy FDA testing and approval process as the original drug. Manufacturers of generic drugs argued that this unfairly stalled competition. The Hatch-Waxman Act introduced the Abbreviated New Drug Application that permits the FDA to approve generic drugs if the applicant can prove its active ingredients are identical to the original drug. This makes generic development more straightforward and less costly. However, the branded manufacturer can still sue for patent infringement, so the law gives generic manufacturers a way to counterbalance the litigation costs. The first generic manufacturer to apply under the process gets a 180-day period when it has exclusive rights to make the generic drug.
Hatch-Waxman also requires branded drug manufacturers to list their patents in an official FDA publication called the “Approved Drug Products with Therapeutic Equivalence,” commonly known as the Orange Book. It identifies patents for small-molecule drugs as well as their approved generic counterparts and the therapeutic equivalence evaluations for the approved drug. This allows doctors, pharmacists, and patients to see if there is an approved generic form of a medicine they are using.
The Biologics Price Competition and Innovation Act was included in the Affordable Care Act and provides an abbreviated approval path for generic versions of large-molecule, biological products, which are produced from living organisms. These include drugs made from a virus or a blood component, such as Avastin, a biologic used by many cancer patients. Because biologic molecules are very complex, making identical products is not possible. The abbreviated pathway allows competitor products to either demonstrate “biosimilarity” or interchangeability with other FDA-approved biologics. The FDA publishes a list of licensed biological products and their approved biosimilar alternatives – the Purple Book.
behavior limiting generic competition
While patent protections are essential to ensuring that inventors will create new treatments for disease, the high cost of prescription drugs has led to concerns that some branded drug companies are using the system to reduce competition. Some of the most common techniques include:
-
Product hopping – A drug creator makes alterations to the original branded or biologic product but with no new substantive therapeutic benefits for patients. By introducing this “new” drug shortly before a patent expires, the manufacturer can force a generic competitor to restart its efforts to have the FDA approve the generic version, allowing the brand version more time without competition.
-
Patent thicketing – Manufacturers may get many patents to cover differences or processes associated with their product. This is intended to make it more difficult for other companies to create a generic form of the drug.
-
Pay-for-delay – The original drug manufacturer negotiates with generic companies to delay launching their alternative products. Typically this happens when a brand-name maker sues a generic company for infringing on its patent.
action in congress
Senate committees have held hearings highlighting the issue of high drug prices and some anti-competitive behavior. In June, the HELP and Judiciary committees advanced bills to combat patent abuse in the pharmaceutical supply chain.
The Lower Health Care Cost Act, was reported out of the HELP Committee on July 8 and includes provisions from 74 senators. One part would allow generic and biosimilar drug makers to sue branded manufacturers to obtain samples required for development and FDA approval. The bill would also update the Orange and Purple books to increase transparency and access for generic manufacturers.
The Judiciary Committee has reported four drug pricing bills. One of these, the Affordable Prescriptions for Patients Act, addresses product hopping and patent-thicketing to make it easier for lower-cost generic alternatives to get to market and spur competition. The bill would allow the Federal Trade Commission to take action against companies that engage in product hopping and would cap the number of patents that can be litigated by a branded biologic manufacturer suing a biosimilar competitor.
Next Article Previous Article
