Responding to Coronavirus
KEY TAKEAWAYS
- Cases of the 2019 coronavirus, recently named COVID-19 by the World Health Organization, have been confirmed in the United States. The virus was first detected in Wuhan, China.
- The situation is evolving rapidly, and the U.S. is responding. Federal agencies are working with state and local health departments to provide test kits, guidelines for health care providers, and screenings to help limit the spread of the virus.
- Congress has provided authority and resources to enable federal agencies to prepare for and respond to public health threats such as this novel coronavirus.
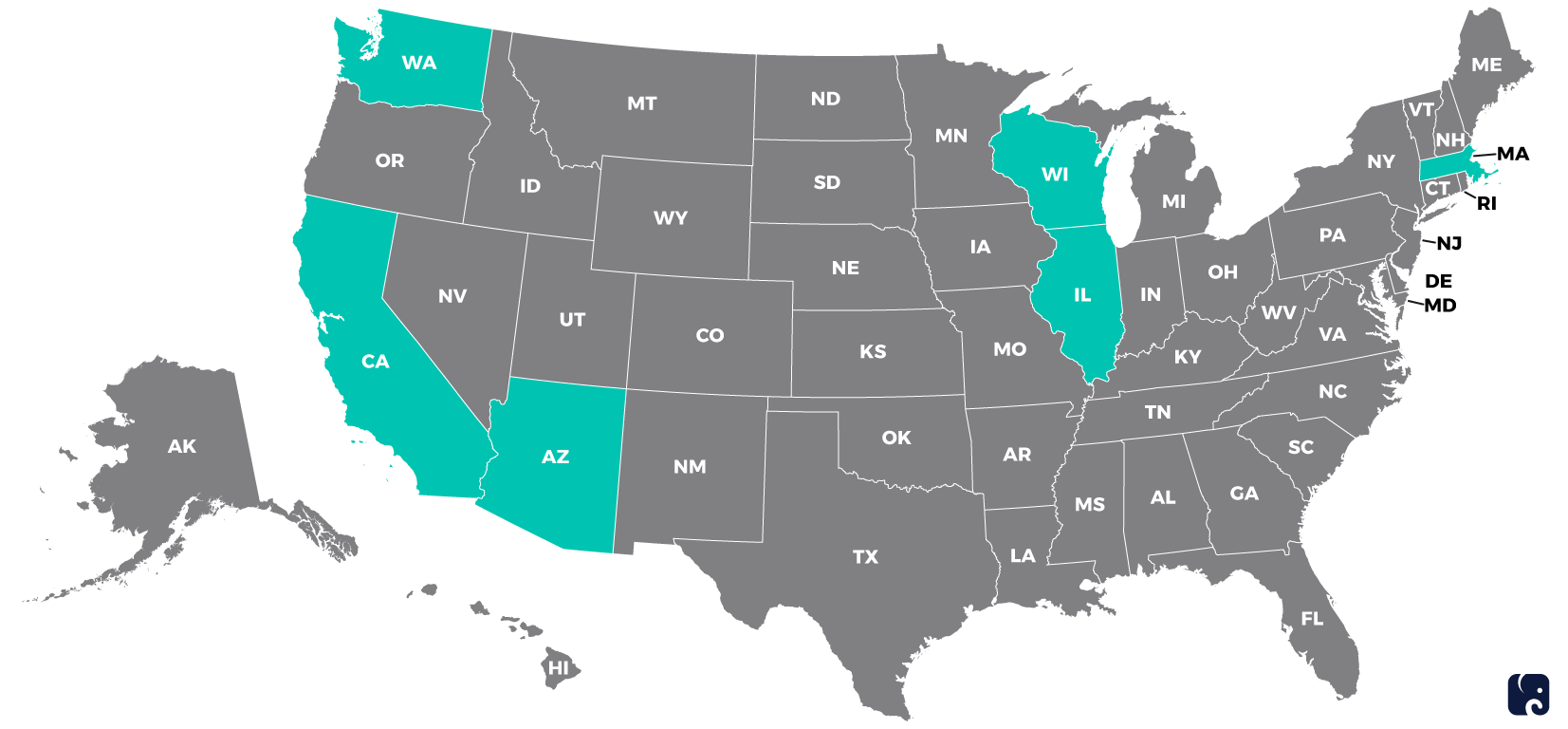
The novel coronavirus, officially named COVID-19, was first identified in Wuhan, China, and reported to the World Health Organization on December 31. It has now spread to more than 20 other countries, including the United States. As of February 12, the Centers for Disease Control and Prevention had identified 14 people in six states who have the virus and was investigating another 66 possible cases. However, the situation is continuing to evolve.
States with Confirmed COVID-19 Cases
On January 31, the administration declared the outbreak to be a public health emergency. This determination allows the federal government to use supplemental resources and deploy public health experts and medical providers to assist in response efforts. President Trump also announced a coronavirus task force on January 29 to coordinate domestic response to the outbreak.
Widespread Response by federal Agencies
The Department of Health and Human Services is leading the federal government response to the virus in the United States, specifically through CDC. The department is monitoring the evolution of the virus closely and is working to limit its spread. As the situation is constantly changing, the department is working with public health officials at state and local levels and providing regular updates to the public. Federal workers have been positioned around the country, including at 11 airports where travel from China has been directed, to aid states in screening, testing, and quarantining travelers as needed.
Key Agencies Involved

The World Health Organization, part of the United Nations, works to promote health around the world and provide global public health assessments and recommendations. Following WHO’s determination that this coronavirus outbreak constituted a “public health emergency of international concern,” the State Department issued a travel advisory on January 31, urging Americans to avoid travel to China. CDC and U.S. Customs and Border Protection have stationed staff at entry points, including airports, to identify and screen travelers who may be at risk of spreading the virus in the United States.
CDC has quarantine and isolation authority to help prevent the spread of diseases in the United States. The agency is requiring people returning to the United States from China to be quarantined for 14 days, the incubation period for the virus, after returning home. The Defense Department has said that it will provide housing for up to 1,000 Americans evacuated from China while they are quarantined. This is the first time in more than 50 years CDC has used its quarantine authority. States and localities may use their own authority to require isolation for sick patients and to quarantine people who may have been exposed.
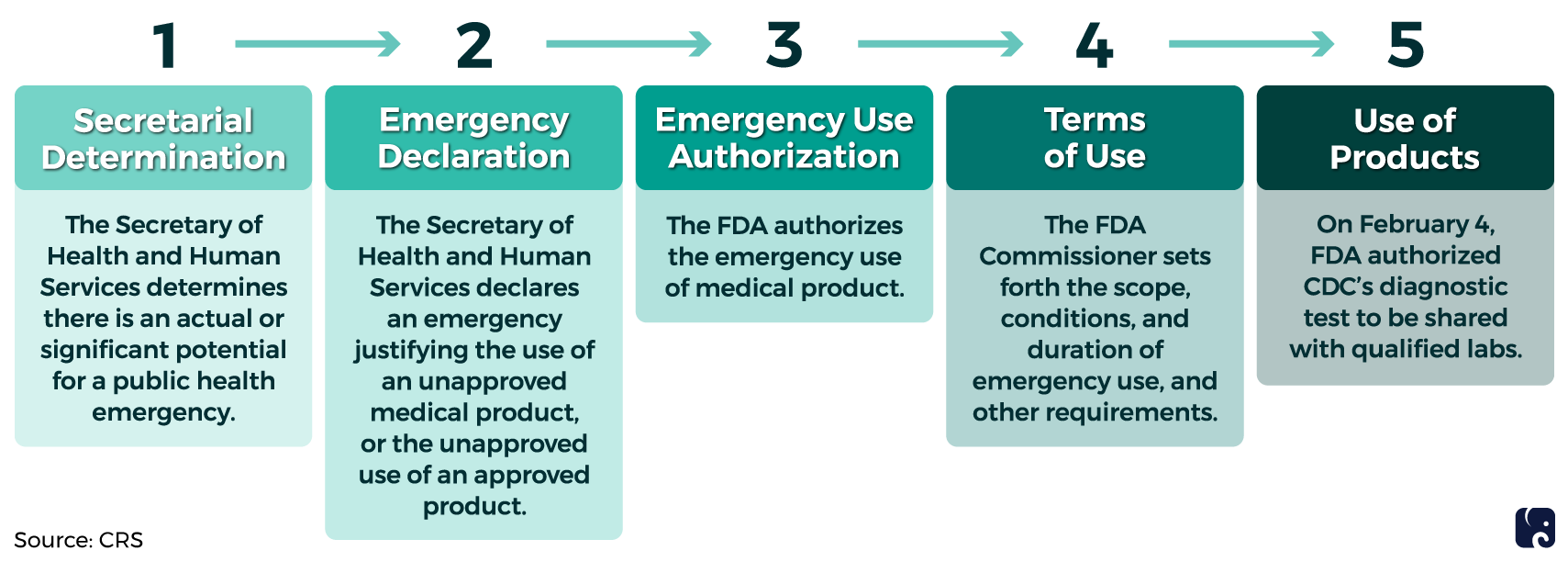
CDC works with state and local health departments, providing them with guidance for screening, testing, and care for patients with infectious diseases. The agency is overseeing the testing of COVID-19 patients in the United States. At the start of the outbreak, CDC developed a diagnostic test at its headquarters in Atlanta. The agency submitted the test to the Food and Drug Administration for “emergency use authorization,” which if granted, allows CDC to provide the test to other laboratories. FDA granted the authorization on February 4, so samples can now be tested in other locations to identify cases more quickly and help slow the spread of the virus.
Emergency Use Authorization Process
U.S. equipped to respond
Congress has equipped health departments and agencies with authority and resources to respond to all hazards, including public health threats. In fiscal year 2019, Congress established the Infectious Disease Rapid Response Reserve Fund to allow HHS to respond to emerging infectious diseases. The administration said on January 25 that it will use the $105 million in this fund for response activities such as airport screening, quarantine facilities, diagnostic tests, and treatment supplies.
Congress appropriated significant resources for preparedness and response at HHS for fiscal year 2020. The Strategic National Stockpile received $705 million, an increase of $95 million, to make sure Americans have access to lifesaving pharmaceuticals and medical supplies. Congress provided $183 million to CDC to help other countries respond to infectious disease outbreaks, an increase of $75 million. Preparing other countries to respond is critical to preventing and managing outbreaks in the U.S.
The National Institute of Allergy and Infectious Diseases, part of NIH, is supporting research on the COVID-19 virus and working with private companies to develop vaccines and potential treatments. These include existing FDA-approved medicines that might be effective against this coronavirus. Congress provided $5.9 billion to NIAID for fiscal year 2020, an increase of $318 million. NIH expects that phase one trials for a vaccine could start within the next few months, though it would take at least a year for a vaccine to be ready to use against this virus.
The Pandemic and All-Hazards Preparedness and Advancing Innovation Act, passed by Congress and signed into law last year, improved America’s preparedness and response programs. The law updated the Public Health Emergency Fund so it can respond more flexibly to outbreaks. HHS also has authority to develop and purchase medical products needed to respond to public health threats.
Next Article Previous Article
