COVID-19 Testing Efforts
KEY TAKEAWAYS
- The Trump administration and Congress have been working to increase coronavirus testing, both diagnostic and antibody testing, to give Americans the confidence to return to work and school.
- Congress passed legislation to increase testing capabilities, including an initiative at the NIH to identify new technologies that can increase diagnostic testing.
- More than 18 million coronavirus tests have been administered in the United States to detect the virus, and the federal government is helping states increase testing.
The Trump administration and Congress have been working on increasing testing rates for COVID-19 to slow the spread of the outbreak and give Americans the peace of mind to return to work and school. Public health experts have reiterated throughout the pandemic that we will need adequate testing to contain the spread of the virus and mitigate its resurgence as states and localities reopen.
enhancing testing capacity
On April 24, President Trump signed the Paycheck Protection Program and Health Care Enhancement Act, which provided $25 billion for testing research and production efforts. Of this amount, Congress appropriated $11 billion to states and Indian health programs for testing support, including contact tracing and surveillance. The amount of for each state is based partly on a formula for the Public Health Emergency Preparedness Grants and partly on the prevalence of the outbreak. This money was on top of at least $2.5 billion that had already been sent to states that could be used for testing, contact tracing, and surveillance.
Testing for COVID-19 – to either diagnose current infection or confirm previous exposure to the virus – is considered an important step in helping to fully reopen much of the country. A diagnostic test tells a patient if he or she has a current infection, and coronavirus diagnostic testing rates are ramping up across the country. Because a person may have the virus without showing symptoms, people may need to be tested more than once. The Department of Health and Human Services is working with retail stores and pharmacies to give people convenient access to testing.
A second type of test, a serological antibody test, can identify if a person has had an immune response to a previous COVID-19 infection. Some studies suggest that a person who has recovered from COVID-19 exposure will likely produce antibodies to the SARS-CoV-2 virus in their blood. Scientists have yet to establish how much protection these antibodies provide against getting infected again, or how long any protection would last, though typically antibodies do confer some immunity against other viruses. Since many cases of the disease are mild or symptom-free, antibody testing may show the pervasiveness of the virus and give officials an indication of whether it is safe to relax more of the restrictions on daily life. Several states have already began surveying their populations for antibodies. There have been questions about the accuracy of antibody tests, and the FDA updated its policies and released a list of tests that do not meet FDA standards for reliability and accuracy.

The Families First and Coronavirus Relief Act – as amended by the Coronavirus Aid, Relief, and Economic Security Act – requires insurers to cover testing to diagnose or detect COVID-19 without any out-of-pocket costs for the patient. On April 11, the Centers for Medicare and Medicaid Services issued guidance confirming commercial health plans and employer-sponsored insurance must cover testing, including antibody testing. Additionally, some insurers and employers who are self-insured have waived deductibles and cost-sharing requirements for coronavirus-related care. The law also ensures testing coverage for Medicare and Medicaid beneficiaries. The federal government also has taken steps to make it easier for people with high-deductible insurance plans to receive testing and treatment before reaching their deductibles.
According to Johns Hopkins University, the United States had tested more than 18 million Americans for the virus. All laboratories must report COVID-19 positive cases to state health departments, and all results are reported to the Centers for Disease Control and Prevention to help researchers get a sense of how and where COVID-19 may be spreading. States are responsible for coming up with testing plans, and the federal government is helping to provide testing supplies. HHS and the Federal Emergency Management Agency sourced and provided more than 13 million swabs and other resources for testing in May, and HHS plans to purchase another 200 million swabs and tubes of viral transport media for states through December. The administration expects these effort will support 40-50 million tests per month by September.
Developing new tests
FDA has granted emergency use authorization for more than 100 tests, some collecting nasal or saliva samples and offering results within days. Guidance issued during the public health emergency has changed the standard process for EUA evaluations at the FDA for the purpose of accelerating testing development.
The Paycheck Protection Program and Health Care Enhancement Act also provided $1 billion for a new initiative led by the National Institutes of Health: the Rapid Acceleration of Diagnostics program. RADx was launched to fast-track the approval of new diagnostic tests that will be easy to use and affordable. Currently, diagnostic tests are being analyzed in labs. The RADx program will fund the development of tests that people can use in their doctor’s office or at home and get reliable results quickly.
RADx Initiative for COVID-19
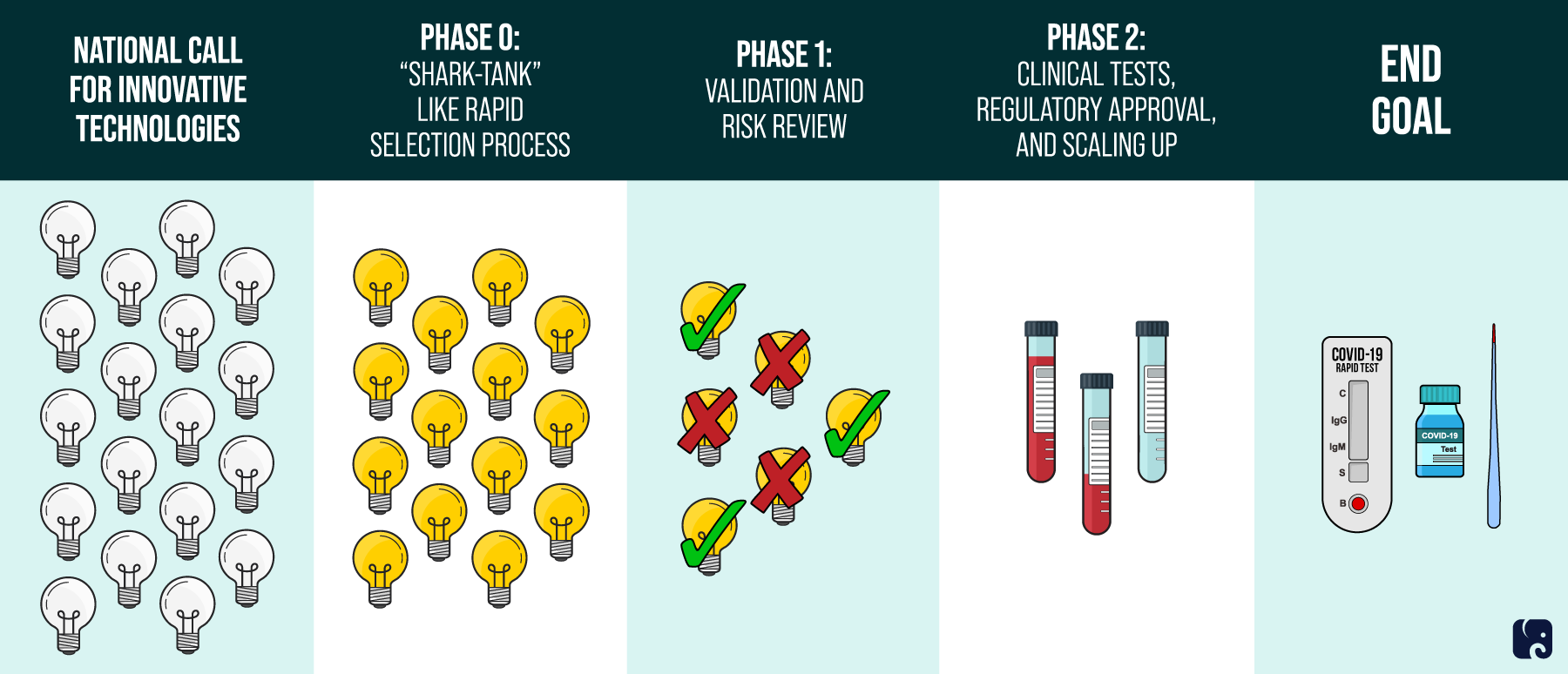
To speed up the creation of these tests, the federal government is working with the private sector and will review potential candidates through a three-phase “shark tank” process. Congress also provided the Biomedical Advanced Research and Development Authority at HHS $1 billion to coordinate with NIH and invest in scaling up these new testing technologies. The FDA will validate diagnostic tests that are produced through the RADx program and will give the tests either emergency use authorization or traditional approval. In the first month RADx was accepting submissions, more than 2,000 applicants expressed interest.
Next Article Previous Article
